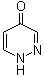
4(1H)-Pyridazinone | CAS:17417-57-1
4(1H)-Pyridazinone
- 名称:4(1H)-哒嗪酮 | 4(1H)-Pyridazinone
- CAS号:17417-57-1
- 别名:
- 分子式:C4H4N2O
- 分子量:96.09
- EINESC号:
产品描述
物理化学性质
| 熔点 | 252 ºC** |
|---|---|
| 沸点 | 184.7±23.0 ºC (760 Tor |
| 闪点 | 65.5±22.6 ºC, 计算值* |
| 密度 | 1.28±0.1 g/cm3 (20 ºC |
安全数据
| 没有数据 | 没有数据 |
|---|
SDS
| 来源 | SDS样本 |
|---|---|
| 没有数据 | 没有数据 |
产品合成路线
| 名称 | CAS号 |
|---|
更多
文章
同行评议文献
Formulation and evaluation of non-effervescent floating tablets of losartan potassium.Anil Getyala et al.Current drug delivery, 10(5), 620-629 (2013-01-05)
In Situ Polyphosphate Nanoparticle Formation in Hybrid Poly(vinyl alcohol)/Karaya Gum Hydrogels: A Porous Scaffold Inducing Infiltration of Mesenchymal Stem Cells.Emad Tolba et al.Advanced science (Weinheim, Baden-Wurttemberg, Germany), 6(2), 1801452-1801452 (2019-01-30)
In vivo evaluation of modified gum karaya as a carrier for improving the oral bioavailability of a poorly water-soluble drug, nimodipine.Gedela V Murali Mohan Babu et al.AAPS PharmSciTech, 3(2), E12-E12 (2003-08-15)
Utility of in situ sodium alginate/karaya gum gels to facilitate gastric retention in rodents.Kimberly A Foster et al.International journal of pharmaceutics, 434(1-2), 406-412 (2012-06-14)
Physical properties of gum karaya-starch-essential oil patches.Yulia Shcherbina et al.AAPS PharmSciTech, 11(3), 1276-1286 (2010-08-17)
Exudate gums: occurrence, production, and applications.D Verbeken et al.Applied microbiology and biotechnology, 63(1), 10-21 (2003-06-13)
Effects of the method of preparation on the compression, mechanical, and release properties of khaya gum matrices.Oluwatoyin A Odeku et al.Pharmaceutical development and technology, 11(4), 435-441 (2006-11-15)
Evaluation of modified gum karaya as carrier for the dissolution enhancement of poorly water-soluble drug nimodipine.G V Murali Mohan Babu et al.International journal of pharmaceutics, 234(1-2), 1-17 (2002-02-13)
Modification of sterculia gum with methacrylic acid to prepare a novel drug delivery system.Baljit Singh et al.International journal of biological macromolecules, 43(2), 142-150 (2008-05-27)
Gastroretentive drug delivery system of metoclopramide hydrochloride: formulation and in vitro evaluation.S Singh et al.Current drug delivery, 4(4), 269-275 (2007-11-06)
Karaya root saponin exerts a hypocholesterolemic response in rats fed a high-cholesterol diet.Sadia Afrose et al.Nutrition research (New York, N.Y.), 29(5), 350-354 (2009-06-27)
[Analysis of thickening polysaccharides by the improved diethyldithioacetal derivatization method].Takumi Akiyama et al.Shokuhin eiseigaku zasshi. Journal of the Food Hygienic Society of Japan, 52(1), 40-46 (2011-03-09)
In vitro efficacy of antimicrobial wafers against methicillin-resistant Staphylococcus aureus.Olga Labovitiadi et al.Therapeutic delivery, 3(4), 443-455 (2012-07-28)
Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application.Vinod Vellora Thekkae Padil et al.International journal of nanomedicine, 8, 889-898 (2013-03-08)
Flow behavior characteristics of ice cream mix made with buffalo milk and various stabilizers.Kuldip S Minhas et al.Plant foods for human nutrition (Dordrecht, Netherlands), 57(1), 25-40 (2002-02-22)
The effect of denture adhesives on Candida albicans growth in vitro.Benedita Sampaio-Maia et al.Gerodontology, 29(2), e348-e356 (2011-04-05)
Lyophilised wafers as vehicles for the topical release of chlorhexidine digluconate--release kinetics and efficacy against Pseudomonas aeruginosa.Olga Labovitiadi et al.International journal of pharmaceutics, 439(1-2), 157-164 (2012-10-23)
Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation.Nikolaos K Andrikopoulos et al.Phytotherapy research : PTR, 17(5), 501-507 (2003-05-16)
Outbreak of cutaneous Rhizopus arrhizus infection associated with karaya ostomy bags.Mysheika LeMaile-Williams et al.Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 43(9), e83-e88 (2006-10-10)
Nimesulide-modified gum karaya solid mixtures: preparation, characterization, and formulation development.G V Murali Mohan Babu et al.Drug development and industrial pharmacy, 29(8), 855-864 (2003-10-23)
Evaluation of Sterculia foetida gum as controlled release excipient.Amit Ashok Chivate et al.AAPS PharmSciTech, 9(1), 197-204 (2008-05-01)
Compressed xanthan and karaya gum matrices: hydration, erosion and drug release mechanisms.D L Munday et al.International journal of pharmaceutics, 203(1-2), 179-192 (2000-09-01)
Evaluation of selected polysaccharide excipients in buccoadhesive tablets for sustained release of nicotine.Calum R Park et al.Drug development and industrial pharmacy, 30(6), 609-617 (2004-08-03)
Development of Denticap, a matrix based sustained release formulation for treatment of toothache, dental infection and other gum problem.Biswajit Mukherjee et al.Current drug delivery, 6(2), 199-207 (2009-05-20)
Development and evaluation of a multiple-unit oral sustained release dosage form for S(+)-ibuprofen: preparation and release kinetics.P J Cox et al.International journal of pharmaceutics, 193(1), 73-84 (1999-12-03)
Suppressive effect of viscous dietary fiber on elevations of uric acid in serum and urine induced by dietary RNA in rats is associated with strength of viscosity.Takashi Koguchi et al.International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition, 73(5), 369-376 (2003-12-03)
Oil-entrapped sterculia gum-alginate buoyant systems of aceclofenac: development and in vitro evaluation.Pravat Ranjan Guru et al.Colloids and surfaces. B, Biointerfaces, 104, 268-275 (2013-01-22)
备注
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).
| 名称 | CAS号 |
|---|

相关产品
相关资讯
问
热门标签
询 单
请 填 写 相 关 信 息
- 请填写产品名称!
- CAS号
- 请正确填写公司名称!
- 请正确填写联系人!
- 请正确填写联系电话!
- 请正确填写联系邮箱!
- 请描述您的问题!