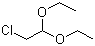
Chloroacetaldehyde diethyl acetal | CAS:621-62-5
Chloroacetaldehyde diethyl acetal
- 名称:氯乙醛缩二乙醇; 2-氯-1,1-二乙氧基乙烷 | Chloroacetaldehyde diethyl acetal
- CAS号:621-62-5
- 别名:2-Chloro-1,1-diethoxyethane
- 分子式:C6H13ClO2
- 分子量:152.62
- EINESC号:210-695-4
产品描述
物理化学性质
| 熔点 | -45 ºC |
|---|---|
| 水溶性 | 1.8 g/L (20 ºC) |
| 沸点 | 157 ºC |
| 闪点 | 29 ºC |
| 密度 | 1.018 |
| 折射率 | 1.415-1.417 |
安全数据
| 危险品标志 | Xi |
|---|---|
| 危险类别码 | R10;R36/37/38 |
| 安全说明书 | S16;S26;S28A |
产品合成路线
| 名称 | CAS号 |
|---|
更多
文章
同行评议文献
Metabolism of methoxyphenamine in vitro by a CYP2D6 microsomal preparation.R T Coutts et al.Drug metabolism and disposition: the biological fate of chemicals, 22(5), 756-760 (1994-09-01)
Effect of sparteine and quinidine on the metabolism of methoxyphenamine by Cunninghamella bainieri.B C Foster et al.Xenobiotica; the fate of foreign compounds in biological systems, 19(4), 445-452 (1989-04-01)
HPLC determination of aminophylline, methoxyphenamine hydrochloride, noscapine and chlorphenamine maleate in compound dosage forms with an aqueous-organic mobile phase.Chunhua Yin et al.Journal of pharmaceutical and biomedical analysis, 33(1), 39-43 (2003-08-30)
Anti-inflammatory effect of methoxyphenamine compound in rat model of chronic obstructive pulmonary disease.Yue-Hong Wang et al.Acta pharmacologica Sinica, 24(12), 1324-1327 (2003-12-05)
Methoxyphenamine inhibits histamine-induced bronchoconstriction in anaesthetized guinea-pigs and histamine-induced contractions of guinea-pig ileum in vitro.J K Callaway et al.Archives internationales de pharmacodynamie et de therapie, 308, 86-94 (1990-11-01)
Selective in vivo inhibition by quinidine of methoxyphenamine oxidation in rat models of human debrisoquine polymorphism.G Muralidharan et al.Xenobiotica; the fate of foreign compounds in biological systems, 19(2), 189-197 (1989-02-01)
The influence of pregnancy on the biotransformation and urinary excretion of methoxyphenamine in mice.B C Foster et al.Biopharmaceutics & drug disposition, 16(1), 1-11 (1995-01-01)
Novel separation scheme for capillary electrophoresis of enantiomers.A Guttman Electrophoresis, 16(10), 1900-1905 (1995-10-01)
Kinetics of in vitro metabolism of methoxyphenamine in rats.S D Roy Xenobiotica; the fate of foreign compounds in biological systems, 20(1), 55-70 (1990-01-01)
Analysis of ethambutol and methoxyphenamine by capillary electrophoresis with electrochemiluminescence detection.Yi-Chien Hsieh et al.Journal of chromatography. A, 1122(1-2), 279-282 (2006-06-27)
Quinidine but not quinine inhibits in man the oxidative metabolic routes of methoxyphenamine which involve debrisoquine 4-hydroxylase.G Muralidharan et al.European journal of clinical pharmacology, 41(5), 471-474 (1991-01-01)
Influence of urinary pH on the disposition of methoxyphenamine and three metabolites in humans.S D Roy et al.Journal of pharmaceutical sciences, 76(6), 427-432 (1987-06-01)
Methoxyphenamine inhibits basal and histamine-induced nasal congestion in anaesthetized rats.W A Lau et al.British journal of pharmacology, 101(2), 394-398 (1990-10-01)
Hair analysis for drugs of abuse. VI. The excretion of methoxyphenamine and methamphetamine into beards of human subjects.Y Nakahara et al.Forensic science international, 63(1-3), 109-119 (1993-12-01)
Development of an LC-MS/MS method for determining 5-MeO-DIPT in dried urine spots and application to forensic cases.Xiuying Yan et al.Journal of forensic and legal medicine, 72, 101963-101963 (2020-05-27)
Efficacy and safety of modified sequential three-step empirical therapy for chronic cough.Weili Wei et al.Respirology (Carlton, Vic.), 15(5), 830-836 (2010-06-16)
The effect of adrenergic agonists and antagonists on cysteine-proteinase inhibitor (cystatin) in rat saliva.G S Bedi Archives of oral biology, 36(8), 611-618 (1991-01-01)
Doping control analysis of methoxyphenamine using liquid chromatography-tandem mass spectrometry.Mario Thevis et al.European journal of mass spectrometry (Chichester, England), 14(3), 145-152 (2008-08-19)
Analysis of benzene ethylamine derivatives in urine using the programmable dynamic liquid-phase microextraction (LPME) device.Seung-Woon Myung et al.The Analyst, 128(12), 1443-1446 (2004-01-23)
Quinine is a more potent inhibitor than quinidine in rat of the oxidative metabolic routes of methoxyphenamine which involve debrisoquine 4-hydroxylase.G Muralidharan et al.Xenobiotica; the fate of foreign compounds in biological systems, 21(11), 1441-1450 (1991-11-01)
Metabolism of methoxyphenamine and 2-methoxyamphetamine in P4502D6-transfected cells and cell preparations.S Geertsen et al.Xenobiotica; the fate of foreign compounds in biological systems, 25(9), 895-906 (1995-09-01)
Enantioselective gas chromatographic assays with electron-capture detection for methoxyphenamine and its three primary metabolites in human urine.N R Srinivas et al.Journal of chromatography, 487(1), 61-72 (1989-01-27)
Microchip-based liquid-liquid extraction for gas-chromatography analysis of amphetamine-type stimulants in urine.Hajime Miyaguchi et al.Journal of chromatography. A, 1129(1), 105-110 (2006-07-29)
Metabolism of methoxyphenamine in extensive and poor metabolizers of debrisoquin.S D Roy et al.Clinical pharmacology and therapeutics, 38(2), 128-133 (1985-08-01)
Separation of optical isomers of methoxyphenamine and its metabolites as N-heptafluorobutyryl-L-prolyl derivatives by fused-silica capillary gas chromatography.S D Roy et al.Journal of chromatography, 431(1), 210-215 (1988-09-23)
Hair analysis for drugs of abuse. III. Movement and stability of methoxyphenamine (as a model compound of methamphetamine) along hair shaft with hair growth.Y Nakahara et al.Journal of analytical toxicology, 16(4), 253-257 (1992-07-01)
备注
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).
| 名称 | CAS号 |
|---|

相关产品
相关资讯
问
热门标签
询 单
请 填 写 相 关 信 息
- 请填写产品名称!
- CAS号
- 请正确填写公司名称!
- 请正确填写联系人!
- 请正确填写联系电话!
- 请正确填写联系邮箱!
- 请描述您的问题!