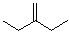
3-Methylenepentane | CAS:760-21-4
3-Methylenepentane
- 名称:3-亚甲基戊烷 | 3-Methylenepentane
- CAS号:760-21-4
- 别名:1,1-Diethylethene; 1,1-Diethylethylene; 2-Ethyl-1-butene; NSC 74120
- 分子式:C6H12
- 分子量:84.16
- EINESC号:212-078-5
产品描述
物理化学性质
| 熔点 | -131.5 ºC** |
|---|---|
| 沸点 | 277-280 ºC* |
| 闪点 | -26.1±0.0 ºC, 计算值*** |
| 密度 | 1.028 g/cm3 (21 ºC)* |
| 折射率 | 1.606 (589.3 nm 21 ºC)* |
安全数据
| 没有数据 | 没有数据 |
|---|
SDS
| 来源 | SDS样本 |
|---|---|
| 没有数据 | 没有数据 |
产品合成路线
| 名称 | CAS号 |
|---|
更多
文章
同行评议文献
In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS).N Thomas et al.Journal of controlled release : official journal of the Controlled Release Society, 160(1), 25-32 (2012-03-13)
In vitro properties of surface-modified solid lipid microspheres containing an antimalarial drug: halofantrine.Anthony A Attama et al.Asian Pacific journal of tropical medicine, 4(4), 253-258 (2011-07-21)
Effects of anti-malarial drugs on the electrocardiographic QT interval modelled in the isolated perfused guinea pig heart system.Atsushi Kinoshita et al.Malaria journal, 9, 318-318 (2010-11-12)
In vitro activities of quinine and other antimalarials and pfnhe polymorphisms in Plasmodium isolates from Kenya.John Okombo et al.Antimicrobial agents and chemotherapy, 54(8), 3302-3307 (2010-06-03)
Is halofantrine ototoxic? Experimental study on guinea pig cochlea model.N M Iskander et al.The Journal of laryngology and otology, 124(10), 1061-1066 (2010-06-12)
Targeted drug delivery to lymphocytes: a route to site-specific immunomodulation?Natalie L Trevaskis et al.Molecular pharmaceutics, 7(6), 2297-2309 (2010-10-21)
Effects of polysorbate 80 on the in-vitro precipitation and oral bioavailability of halofantrine from polyethylene glycol 400 formulations in rats.Henrik Tønsberg et al.The Journal of pharmacy and pharmacology, 62(1), 63-70 (2010-08-21)
Metabolic network analysis predicts efficacy of FDA-approved drugs targeting the causative agent of a neglected tropical disease.Arvind K Chavali et al.BMC systems biology, 6, 27-27 (2012-05-01)
Structural analysis of the antimalarial drug halofantrine by means of Raman spectroscopy and density functional theory calculations.Torsten Frosch et al.Journal of biomedical optics, 15(4), 041516-041516 (2010-08-31)
CDA: combinatorial drug discovery using transcriptional response modules.Ji-Hyun Lee et al.PloS one, 7(8), e42573-e42573 (2012-08-21)
Influence of bile on the absorption of halofantrine from lipid-based formulations.René Holm et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 81(2), 281-287 (2012-04-03)
Effect of serum lipoproteins on stereoselective halofantrine metabolism by rat hepatocytes.Jigar P Patel et al.Chirality, 24(7), 558-565 (2012-05-17)
Measurement of lumefantrine and its metabolite in plasma by high performance liquid chromatography with ultraviolet detection.Insaf F Khalil et al.Journal of pharmaceutical and biomedical analysis, 54(1), 168-172 (2010-09-14)
Population pharmacokinetics of halofantrine in healthy volunteers and patients with symptomatic falciparum malaria.Kerenaftali Klein et al.The Journal of pharmacy and pharmacology, 64(11), 1603-1613 (2012-10-13)
Acute hypertriglyceridemia promotes intestinal lymphatic lipid and drug transport: a positive feedback mechanism in lipid and drug absorption.Natalie L Trevaskis et al.Molecular pharmaceutics, 8(4), 1132-1139 (2011-05-25)
Distribution of drug resistance genotypes in Plasmodium falciparum in an area of limited parasite diversity in Saudi Arabia.Saad M Bin Dajem et al.The American journal of tropical medicine and hygiene, 86(5), 782-788 (2012-05-05)
Intravenous dosing conditions may affect systemic clearance for highly lipophilic drugs: implications for lymphatic transport and absolute bioavailability studies.Suzanne M Caliph et al.Journal of pharmaceutical sciences, 101(9), 3540-3546 (2012-05-25)
Effect of halofantrine on QT interval in children.Jean-Yves Siriez et al.Pathogens and global health, 106(2), 124-125 (2012-09-05)
Discordant temporal evolution of Pfcrt and Pfmdr1 genotypes and Plasmodium falciparum in vitro drug susceptibility to 4-aminoquinolines after drug policy change in French Guiana.Eric Legrand et al.Antimicrobial agents and chemotherapy, 56(3), 1382-1389 (2012-01-11)
Effect of experimental hyperlipidaemia on the electrocardiographic effects of repeated doses of halofantrine in rats.Jigar P Patel et al.British journal of pharmacology, 161(6), 1427-1440 (2010-08-12)
Effect of bile on the oral absorption of halofantrine in polyethylene glycol 400 and polysorbate 80 formulations dosed to bile duct cannulated rats.Henrik Tønsberg et al.The Journal of pharmacy and pharmacology, 63(6), 817-824 (2011-05-19)
Using resin to generate a non-invasive intestinal bile-depleted rat model was unsuccessful.René Holm et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 47(2), 347-351 (2012-06-27)
A novel excipient, 1-perfluorohexyloctane shows limited utility for the oral delivery of poorly water-soluble drugs.René Holm et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 42(4), 416-422 (2011-01-25)
In vitro susceptibility of Plasmodium falciparum to monodesethylamodiaquine, quinine, mefloquine and halofantrine in Abidjan (Côte d'Ivoire).W Yavo et al.African health sciences, 10(2), 111-116 (2011-02-18)
Identification and functional validation of the novel antimalarial resistance locus PF10_0355 in Plasmodium falciparum.Daria Van Tyne et al.PLoS genetics, 7(4), e1001383-e1001383 (2011-05-03)
备注
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).
| 名称 | CAS号 |
|---|

相关产品
相关资讯
问
热门标签
询 单
请 填 写 相 关 信 息
- 请填写产品名称!
- CAS号
- 请正确填写公司名称!
- 请正确填写联系人!
- 请正确填写联系电话!
- 请正确填写联系邮箱!
- 请描述您的问题!