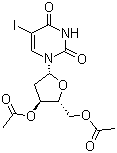
3',5'-Di-O-acetyl-5-iodo-2'-deoxyuridine | CAS:1956-30-5
3',5'-Di-O-acetyl-5-iodo-2'-deoxyuridine
- 名称:3',5'-二-O-乙酰基-5-碘-2'-脱氧尿苷 | 3',5'-Di-O-acetyl-5-iodo-2'-deoxyuridine
- CAS号:1956-30-5
- 别名:
- 分子式:C13H15IN2O7
- 分子量:438.17
- EINESC号:217-799-9
产品描述
物理化学性质
| 熔点 | 156-158 ºC |
|---|---|
| 密度 | 1.81 |
安全数据
| 没有数据 | 没有数据 |
|---|
SDS
| 来源 | SDS样本 |
|---|---|
| 没有数据 | 没有数据 |
产品合成路线
| 名称 | CAS号 |
|---|
更多
文章
同行评议文献
Investigation of the potential for direct compaction of a fine ibuprofen powder dry-coated with magnesium stearate.Li Qu et al.Drug development and industrial pharmacy, 41(5), 825-837 (2014-04-18)
Development and optimization of taste-masked orally disintegrating tablets (ODTs) of clindamycin hydrochloride.Stuart L Cantor et al.Drug development and industrial pharmacy, 41(7), 1156-1164 (2014-07-08)
Determination of counterfeit medicines by Raman spectroscopy: Systematic study based on a large set of model tablets.Sabine Neuberger et al.Journal of pharmaceutical and biomedical analysis, 112, 70-78 (2015-05-10)
Continuous twin screw melt granulation of glyceryl behenate: Development of controlled release tramadol hydrochloride tablets for improved safety.Justin M Keen et al.International journal of pharmaceutics, 487(1-2), 72-80 (2015-04-04)
Concurrent oral and inhalation drug delivery using a dual formulation system: the use of oral theophylline carrier with combined inhalable budesonide and terbutaline.Rania O Salama et al.Drug delivery and translational research, 4(3), 256-267 (2015-03-20)
Gastroretentive Ranitidine Hydrochloride Tablets with Combined Floating and Bioadhesive Properties: Factorial Design Analysis, In Vitro Evaluation and In Vivo Abdominal X-Ray Imaging.Hana N Abduljabbar et al.Current drug delivery, 12(5), 578-590 (2015-06-09)
Fed and fasted state gastro-intestinal in vitro lipolysis: In vitro in vivo relations of a conventional tablet, a SNEDDS and a solidified SNEDDS.Philip Carsten Christophersen et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 57, 232-239 (2013-09-24)
Commercial scale validation of a process scale-up model for lubricant blending of pharmaceutical powders.Joseph Kushner et al.International journal of pharmaceutics, 475(1-2), 147-155 (2014-08-26)
Preparation and evaluation of ritodrine buccal tablets for rational therapeutic use.Hiraku Onishi et al.International journal of pharmaceutics, 468(1-2), 207-213 (2014-04-09)
Gastro-resistant characteristics of GRAS-grade enteric coatings for pharmaceutical and nutraceutical products.Justyna K Czarnocka et al.International journal of pharmaceutics, 486(1-2), 167-174 (2015-03-23)
Influence of coating material on the flowability and dissolution of dry-coated fine ibuprofen powders.Li Qu et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 78, 264-272 (2015-07-29)
Film-coated matrix mini-tablets for the extended release of a water-soluble drug.Faiezah A A Mohamed et al.Drug development and industrial pharmacy, 41(4), 623-630 (2014-02-26)
Formulation and evaluation of different floating tablets containing metronidazole to target stomach.Zhiao C Loh et al.Current drug delivery, 12(4), 425-443 (2015-05-01)
Adrenaline (epinephrine) microcrystal sublingual tablet formulation: enhanced absorption in a preclinical model.Mutasem Rawas-Qalaji et al.The Journal of pharmacy and pharmacology, 67(1), 20-25 (2014-09-27)
Can preschool-aged children swallow several minitablets at a time? Results from a clinical pilot study.A Kluk et al.International journal of pharmaceutics, 485(1-2), 1-6 (2015-03-05)
Characterising the disintegration properties of tablets in opaque media using texture analysis.Rebekah L Scheuerle et al.International journal of pharmaceutics, 486(1-2), 136-143 (2015-03-21)
Development and validation of X-ray diffraction method for quantitative determination of crystallinity in warfarin sodium products.Akhtar Siddiqui et al.International journal of pharmaceutics, 493(1-2), 1-6 (2015-07-26)
Preformulation characterization and in vivo absorption in beagle dogs of JFD, a novel anti-obesity drug for oral delivery.Yunzhou Fan et al.Drug development and industrial pharmacy, 41(5), 801-811 (2014-04-04)
New approach to evaluate the lubrication process in various granule filling levels and rotating mixer sizes using a thermal effusivity sensor.Jumpei Uchiyama et al.Chemical & pharmaceutical bulletin, 63(3), 164-179 (2015-03-12)
A menthol-based solid dispersion technique for enhanced solubility and dissolution of sulfamethoxazole from an oral tablet matrix.Bibi F Choonara et al.AAPS PharmSciTech, 16(4), 771-786 (2015-01-01)
Design, development and in-vitro evaluation of diclofenac taste-masked orodispersible tablet formulations.Marie Guhmann et al.Drug development and industrial pharmacy, 41(4), 540-551 (2014-02-06)
Physical characterization of the polymorphic variations of magnesium stearate and magnesium palmitate hydrate species. Sharpe SA, et al. Structural Chemistry, 8(1), 73-84 (1997)
Determining the polymer threshold amount for achieving robust drug release from HPMC and HPC matrix tablets containing a high-dose BCS class I model drug: in vitro and in vivo studies.Uroš Klančar et al.AAPS PharmSciTech, 16(2), 398-406 (2014-10-22)
Preparation and characterisation of Kolliphor® P 188 and P 237 solid dispersion oral tablets containing the poorly water soluble drug disulfiram.Nisrina Ramadhani et al.International journal of pharmaceutics, 475(1-2), 514-522 (2014-09-15)
Applicability of UV laser-induced solid-state fluorescence spectroscopy for characterization of solid dosage forms.Eva Woltmann et al.Analytical and bioanalytical chemistry, 406(25), 6347-6362 (2014-08-12)
Design and evaluation of a specific, bi-phase extended release system based on differently coated mini-tablets.Aleksandar Aleksovski et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 75, 114-122 (2015-04-08)
Rapid quantitative analysis of magnesium stearate in tablets using laser-induced breakdown spectroscopy.Louis St-Onge et al.Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques, 8(2), 272-288 (2005-08-30)
Evaluation of Phosphorylated Psyllium Seed Polysaccharide as a Release Retardant.Monica R P Rao et al.Indian journal of pharmaceutical sciences, 77(5), 605-612 (2016-01-23)
Efficacy of gastro-retentive forms of ecabet sodium in the treatment of gastric ulcer in rats.Ju-Young Kim et al.Archives of pharmacal research, 37(8), 1053-1062 (2013-11-21)
Development of taste masked caffeine citrate formulations utilizing hot melt extrusion technology and in vitro-in vivo evaluations.Manjeet B Pimparade et al.International journal of pharmaceutics, 487(1-2), 167-176 (2015-04-19)
Evaluation of the lubrication mechanism at various rotation speeds and granule filling levels in a container mixer using a thermal effusivity sensor.Jumpei Uchiyama et al.Drug development and industrial pharmacy, 41(7), 1172-1181 (2014-07-08)
Use of hydrophilic natural gums in formulation of sustained-release matrix tablets of tramadol hydrochloride.Jaleh Varshosaz et al.AAPS PharmSciTech, 7(1), E24-E24 (2006-04-06)
A newly developed lubricant, chitosan laurate, in the manufacture of acetaminophen tablets.Ahmad Bani-Jaber et al.International journal of pharmaceutics, 483(1-2), 49-56 (2015-02-11)
Diclofenac sodium multisource prolonged release tablets--a comparative study on the dissolution profiles.Paola Bertocchi et al.Journal of pharmaceutical and biomedical analysis, 37(4), 679-685 (2005-03-31)
Dependence of tablet brittleness on tensile strength and porosity.Xingchu Gong et al.International journal of pharmaceutics, 493(1-2), 208-213 (2015-08-01)
Quantitative correlation of the effect of process conditions on the capping tendencies of tablet formulations.Ilgaz Akseli et al.Journal of pharmaceutical sciences, 103(6), 1652-1663 (2014-03-29)
Anti-inflammatory and pharmacokinetics evaluation of PEGylated ibuprofen tablet formulation.Momoh A Mumuni et al.Drug delivery, 21(4), 315-319 (2013-11-07)
Rapid quantitative analysis of magnesium stearate in tablets using laser-induced breakdown spectroscopy.Louis St-Onge et al.Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques, 8(2), 272-288 (2005-08-30)
Disulfiram-loaded immediate and extended release vaginal tablets for the localised treatment of cervical cancer.Clara S Baffoe et al.The Journal of pharmacy and pharmacology, 67(2), 189-198 (2014-12-17)
The stress stability of olanzapine: studies of interactions with excipients in solid state pharmaceutical formulations.Nataša Djordjević Filijović et al.Drug development and industrial pharmacy, 41(3), 502-514 (2014-03-13)
Bilayer matrix tablets for prolonged actions of metformin hydrochloride and repaglinide.Wei He et al.AAPS PharmSciTech, 16(2), 344-353 (2014-10-17)
Delay effect of magnesium stearate on tablet dissolution in acidic medium.Ariyasu A, et al. International Journal of Pharmaceutics, 511(2), 757-764 (2016)
Use of Drug Release Testing to Evaluate the Retention of Abuse-Deterrent Properties of Polyethylene Oxide Matrix Tablets.Saikishore Meruva et al.AAPS PharmSciTech, 21(7), 270-270 (2020-10-08)
Phosphodiesterase 3 inhibitor cilostazol induces migraine-like attacks via cyclic AMP increase.Song Guo et al.Brain : a journal of neurology, 137(Pt 11), 2951-2959 (2014-08-28)
Formulating powder-device combinations for salmeterol xinafoate dry powder inhalers.Mireille Hassoun et al.International journal of pharmaceutics, 490(1-2), 360-367 (2015-05-20)
VIS-NIR spectroscopy as a process analytical technology for compositional characterization of film biopolymers and correlation with their mechanical properties.Douglas Fernandes Barbin et al.Materials science & engineering. C, Materials for biological applications, 56, 274-279 (2015-08-08)
Evaluating and modifying Johanson's rolling model to improve its predictability.Mingda Bi et al.Journal of pharmaceutical sciences, 103(7), 2062-2071 (2014-05-21)
Controlled release of acidic drugs in compendial and physiological hydrogen carbonate buffer from polymer blend-coated oral solid dosage forms.R Wulff et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 77, 246-253 (2015-06-21)
A new apparatus for real-time assessment of the particle size distribution of disintegrating tablets.Julian Quodbach et al.Journal of pharmaceutical sciences, 103(11), 3657-3665 (2014-09-17)
Wetting effects versus ion pairs diffusivity: interactions of anionic surfactants with highly soluble cationic drugs and its impact on tablet dissolution.Divyakant Desai et al.Journal of pharmaceutical sciences, 104(7), 2255-2265 (2015-05-29)
Densification properties and compactibility of mixtures of pharmaceutical excipients with and without magnesium stearate.Vromans H and Lerk CF International Journal of Pharmaceutics, 46(3), 183-192 (1988)
Antisolvent precipitation of novel xylitol-additive crystals to engineer tablets with improved pharmaceutical performance.Waseem Kaialy et al.International journal of pharmaceutics, 477(1-2), 282-293 (2014-12-03)
Preparation and characterization of Compritol 888 ATO matrix tablets for the sustained release of diclofenac sodium.Matthew Roberts et al.Pharmaceutical development and technology, 20(4), 507-512 (2013-12-21)
Mefenamic acid taste-masked oral disintegrating tablets with enhanced solubility via molecular interaction produced by hot melt extrusion technology.Sultan M Alshehri et al.Journal of drug delivery science and technology, 27, 18-27 (2015-04-29)
Influence of Prosolv and Prosolv:Mannitol 200 direct compression fillers on the physicomechanical properties of atorvastatin oral dispersible tablets.Veeran Gowda et al.Pharmaceutical development and technology, 20(4), 394-400 (2014-01-09)
A new tablet brittleness index.Xingchu Gong et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 93, 260-266 (2015-04-25)
A study of micronized poloxamers as lubricants in direct compression of tablets.Jitka Muzíková et al.Acta poloniae pharmaceutica, 70(6), 1087-1096 (2014-01-05)
Conversion of solid dispersion prepared by acid-base interaction into free-flowing and tabletable powder by using Neusilin® US2.Ankita Shah et al.International journal of pharmaceutics, 484(1-2), 172-180 (2015-03-01)
Tablet mechanics depend on nano and micro scale adhesion, lubrication and structure.Maria Badal Tejedor et al.International journal of pharmaceutics, 486(1-2), 315-323 (2015-04-07)
Predictive evaluation of pharmaceutical properties of direct compression tablets containing theophylline anhydrate during storage at high humidity by near-infrared spectroscopy.Yuta Otsuka et al.Bio-medical materials and engineering, 25(3), 223-236 (2015-09-26)
Determination of tacrolimus crystalline fraction in the commercial immediate release amorphous solid dispersion products by a standardized X-ray powder diffraction method with chemometrics.Ziyaur Rahman et al.International journal of pharmaceutics, 475(1-2), 462-470 (2014-09-01)
Bromocriptine tablet of self-microemulsifying system adsorbed onto porous carrier to stimulate lipoproteins secretion for brain cellular uptake.Sirigul Thongrangsalit et al.Colloids and surfaces. B, Biointerfaces, 131, 162-169 (2015-05-20)
Formulation and in vitro, in vivo evaluation of effervescent floating sustained-release imatinib mesylate tablet.Ali Kadivar et al.PloS one, 10(6), e0126874-e0126874 (2015-06-04)
Assessment of disintegrant efficacy with fractal dimensions from real-time MRI.Julian Quodbach et al.International journal of pharmaceutics, 475(1-2), 605-612 (2014-09-23)
Effect of simulated precompression, compression pressure and tableting speed on an offline diffuse transmittance and reflectance near-infrared spectral information of model intact caffeine tablets.Branko Z Vranic et al.Pharmaceutical development and technology, 20(1), 90-98 (2014-08-15)
Second-derivative synchronous spectrofluorimetric determination of nebivolol hydrochloride and amlodipine besylate in their combined dosage form.F Ibrahim et al.Luminescence : the journal of biological and chemical luminescence, 30(7), 1011-1019 (2015-03-03)
Quality-by-design III: application of near-infrared spectroscopy to monitor roller compaction in-process and product quality attributes of immediate release tablets.Ravikanth Kona et al.AAPS PharmSciTech, 16(1), 202-216 (2014-10-17)
Analysis of Distribution of Ingredients in Commercially Available Clarithromycin Tablets Using Near-Infrared Chemical Imaging with Principal Component Analysis and Partial Least Squares.Tatsuo Koide et al.Chemical & pharmaceutical bulletin, 63(9), 663-668 (2015-09-04)
A Holistic Multi Evidence Approach to Study the Fragmentation Behaviour of Crystalline Mannitol.Jasdip S Koner et al.Scientific reports, 5, 16352-16352 (2015-11-11)
Solid-state stability and compatibility studies of clavulanate potassium.Judyta Cielecka-Piontek et al.Pharmaceutical development and technology, 20(2), 146-152 (2013-11-14)
Controlled-release triple anti-inflammatory therapy based on novel gastroretentive sponges: characterization and magnetic resonance imaging in healthy volunteers.Mina Ibrahim Tadros et al.International journal of pharmaceutics, 472(1-2), 27-39 (2014-06-15)
Lean production of taste improved lipidic sodium benzoate formulations.C Eckert et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 88(2), 455-461 (2014-05-27)
A novel approach to a fine particle coating using porous spherical silica as core particles.Makoto Ishida et al.Drug development and industrial pharmacy, 40(8), 1054-1064 (2013-06-21)
The effect of polymer properties on direct compression and drug release from water-insoluble controlled release matrix tablets.Julia Grund et al.International journal of pharmaceutics, 469(1), 94-101 (2014-04-22)
Evaluation of Phosphorylated Psyllium Seed Polysaccharide as a Release Retardant.Monica R P Rao et al.Indian journal of pharmaceutical sciences, 77(5), 605-612 (2016-01-23)
Efficacy of gastro-retentive forms of ecabet sodium in the treatment of gastric ulcer in rats.Ju-Young Kim et al.Archives of pharmacal research, 37(8), 1053-1062 (2013-11-21)
Development of taste masked caffeine citrate formulations utilizing hot melt extrusion technology and in vitro-in vivo evaluations.Manjeet B Pimparade et al.International journal of pharmaceutics, 487(1-2), 167-176 (2015-04-19)
Evaluation of the lubrication mechanism at various rotation speeds and granule filling levels in a container mixer using a thermal effusivity sensor.Jumpei Uchiyama et al.Drug development and industrial pharmacy, 41(7), 1172-1181 (2014-07-08)
Use of hydrophilic natural gums in formulation of sustained-release matrix tablets of tramadol hydrochloride.Jaleh Varshosaz et al.AAPS PharmSciTech, 7(1), E24-E24 (2006-04-06)
A newly developed lubricant, chitosan laurate, in the manufacture of acetaminophen tablets.Ahmad Bani-Jaber et al.International journal of pharmaceutics, 483(1-2), 49-56 (2015-02-11)
The role of physico-chemical and bulk characteristics of co-spray dried L-leucine and polyvinylpyrrolidone on glidant and binder properties in interactive mixtures.Sharad Mangal et al.International journal of pharmaceutics, 479(2), 338-348 (2015-01-13)
Latent structure modeling underlying theophylline tablet formulations using a Bayesian network based on a self-organizing map clustering.Akihito Yasuda et al.Drug development and industrial pharmacy, 41(7), 1148-1155 (2014-07-06)
Impact of micromeritic properties of an active pharmaceutical ingredient on its compaction behavior.Dilbir S Bindra et al.Pharmaceutical development and technology, 20(2), 129-138 (2013-11-14)
Development of Transmission Raman Spectroscopy towards the in line, high throughput and non-destructive quantitative analysis of pharmaceutical solid oral dose.Julia A Griffen et al.The Analyst, 140(1), 107-112 (2014-11-02)
Critical evaluation of root causes of the reduced compactability after roll compaction/dry granulation.Johanna Mosig et al.Journal of pharmaceutical sciences, 104(3), 1108-1118 (2015-01-07)
Amorphous solid dispersion with increased gastric solubility in tandem with oral disintegrating tablets: a successful approach to improve the bioavailability of atorvastatin.Jumah Masoud M Salmani et al.Pharmaceutical development and technology, 20(4), 465-472 (2014-02-05)
Accelerating the dissolution of enteric coatings in the upper small intestine: evolution of a novel pH 5.6 bicarbonate buffer system to assess drug release.Felipe J O Varum et al.International journal of pharmaceutics, 468(1-2), 172-177 (2014-04-15)
Effect of mixing time on the lubricating properties of magnesium stearate and the final characteristics of the compressed tablets.Kikuta J-I and Kitamori N. Drug Development and Industrial Pharmacy, 20(3), 343-355 (1994)
Solid formulations by a nanocrystal approach: critical process parameters regarding scale-ability of nanocrystals for tableting applications.Annika Tuomela et al.International journal of pharmaceutics, 485(1-2), 77-86 (2015-03-10)
Combination effect of physical and gustatory taste masking for propiverine hydrochloride orally disintegrating tablets on palatability.Rakan Matsui et al.Biological & pharmaceutical bulletin, 38(1), 17-22 (2015-03-07)
Structural changes of polymer-coated microgranules and excipients on tableting investigated by microtomography using synchrotron X-ray radiation.Ryusuke Kajihara et al.International journal of pharmaceutics, 481(1-2), 132-139 (2015-02-11)
Controlled porosity solubility modulated osmotic pump tablets of gliclazide.Arti Banerjee et al.AAPS PharmSciTech, 16(3), 554-568 (2014-11-08)
Evaluation of risk and benefit in thermal effusivity sensor for monitoring lubrication process in pharmaceutical product manufacturing.Jumpei Uchiyama et al.Drug development and industrial pharmacy, 40(8), 999-1004 (2013-05-23)
Preparation and evaluation of oral controlled-release cilostazol formulation: pharmacokinetics and antithrombotic efficacy in dogs and healthy male Korean participants.Kwang-Hyun Shin et al.The Journal of pharmacy and pharmacology, 66(7), 961-974 (2014-04-05)
Evaluating scale-up rules of a high-shear wet granulation process.Jing Tao et al.Journal of pharmaceutical sciences, 104(7), 2323-2333 (2015-05-27)
Effect of process variables on the Drucker-Prager cap model and residual stress distribution of tablets estimated by the finite element method.Yoshihiro Hayashi et al.Chemical & pharmaceutical bulletin, 62(11), 1062-1072 (2014-08-12)
Assay method for quality control and stability studies of a new anti-diabetic and anti-dyslipidemic flavone (S002-853).Arshi Naqvi et al.Pharmacognosy magazine, 11(Suppl 1), S53-S59 (2015-06-26)
备注
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).
| 名称 | CAS号 |
|---|

相关产品
相关资讯
问
热门标签
询 单
请 填 写 相 关 信 息
- 请填写产品名称!
- CAS号
- 请正确填写公司名称!
- 请正确填写联系人!
- 请正确填写联系电话!
- 请正确填写联系邮箱!
- 请描述您的问题!