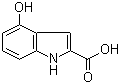
4-Hydroxy-1H-indole-2-carboxylic acid | CAS:80129-52-8
4-Hydroxy-1H-indole-2-carboxylic acid
- 名称:4-羟基吲哚-2-甲酸 | 4-Hydroxy-1H-indole-2-carboxylic acid
- CAS号:80129-52-8
- 别名:
- 分子式:C9H7NO3
- 分子量:177.16
- EINESC号:
产品描述
物理化学性质
| 没有数据 | 没有数据 |
|---|
安全数据
| 没有数据 | 没有数据 |
|---|
SDS
| 来源 | SDS样本 |
|---|---|
| Matrix | 浏览或下载 |
产品合成路线
| 名称 | CAS号 |
|---|
更多
文章
同行评议文献
Ethylcellulose-based matrix-type microspheres: influence of plasticizer RATIO as pore-forming agent.C Tuba Sengel-Turk et al.AAPS PharmSciTech, 12(4), 1127-1135 (2011-09-03)
Stability of dry coated solid dosage forms.Caroline Désirée Kablitz et al.Pharmaceutical development and technology, 14(6), 613-622 (2009-11-04)
The influence of surfactants and additives on drug release from a cationic eudragit coated multiparticulate diltiazem formulation.Grant Heinicke et al.Pharmaceutical development and technology, 12(4), 381-389 (2007-09-01)
Evaluation of the effect of physical variables on in vitro release of diclofenac pellets using Box-Behnken design.Reza Enayatifard et al.Iranian journal of basic medical sciences, 18(7), 710-714 (2015-09-10)
Development and evaluation of chitosan and chitosan/Kollicoat® Smartseal 30 D film-coated tablets for colon targeting.Michael Drechsler et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 88(3), 807-815 (2014-10-11)
Preparation and evaluation of enteric-coated delayed-release pellets of duloxetine hydrochloride using a fluidized bed coater.Yong-Il Kim et al.Archives of pharmacal research, 38(12), 2163-2171 (2015-07-18)
A novel concept in enteric coating: a double-coating system providing rapid drug release in the proximal small intestine.Fang Liu et al.Journal of controlled release : official journal of the Controlled Release Society, 133(2), 119-124 (2008-10-22)
[Effects of wheat leaf surface waxes on the feeding of two wheat aphid species].Yong Liu et al.Ying yong sheng tai xue bao = The journal of applied ecology, 18(8), 1785-1788 (2007-11-03)
Preparation and evaluation of diltiazem hydrochloride diffusion-controlled transdermal delivery system.Ekapol Limpongsa et al.AAPS PharmSciTech, 9(2), 464-470 (2008-04-24)
Development and evaluation of in situ novel intragastric controlled-release formulation of hydrochlorothiazide.Ravikumar R Patel et al.Acta pharmaceutica (Zagreb, Croatia), 61(1), 73-82 (2011-03-17)
Intestinal pregnane X receptor links xenobiotic exposure and hypercholesterolemia.Yipeng Sui et al.Molecular endocrinology (Baltimore, Md.), 29(5), 765-776 (2015-03-27)
Enteric-coating of pulsatile-release HPC capsules prepared by injection molding.E Macchi et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 70, 1-11 (2015-01-15)
Preparation and evaluation of sustained-release matrix tablets based on metoprolol and an acrylic carrier using injection moulding.T Quinten et al.AAPS PharmSciTech, 13(4), 1197-1211 (2012-09-12)
The use of dynamic mechanical analysis (DMA) to evaluate plasticization of acrylic polymer films under simulated gastrointestinal conditions.H M Fadda et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 76(3), 493-497 (2010-08-31)
Real-time predictions of drug release and end point detection of a coating operation by in-line near infrared measurements.Claire Gendre et al.International journal of pharmaceutics, 421(2), 237-243 (2011-10-11)
Characterization of thermal and rheological properties of zidovudine, lamivudine and plasticizer blends with ethyl cellulose to assess their suitability for hot melt extrusion.Shital M Maru et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 44(4), 471-478 (2011-09-20)
Controlled release of acidic drugs in compendial and physiological hydrogen carbonate buffer from polymer blend-coated oral solid dosage forms.R Wulff et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 77, 246-253 (2015-06-21)
Real-time predictions of drug release and end point detection of a coating operation by in-line near infrared measurements.Claire Gendre et al.International journal of pharmaceutics, 421(2), 237-243 (2011-10-11)
Stability of dry coated solid dosage forms.Caroline Désirée Kablitz et al.Pharmaceutical development and technology, 14(6), 613-622 (2009-11-04)
Controlled delivery of a new broad spectrum antibacterial agent against colitis: In vitro and in vivo performance.M S Nieto-Bobadilla et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 96, 152-161 (2015-07-26)
[The mechanical properties and moisture permeability of eudragit L100/S100 free films affected by plasticizers and membrane materials ratio].Guo-song Zhang et al.Yao xue xue bao = Acta pharmaceutica Sinica, 46(9), 1144-1149 (2011-11-30)
Application of quality by design (QbD) to formulation and processing of naproxen pellets by extrusion-spheronization.Junlin Wang et al.Pharmaceutical development and technology, 20(2), 246-256 (2014-07-30)
Double-blind, randomized, placebo-controlled study of a lotion containing triethyl citrate and ethyl linoleate in the treatment of acne vulgaris.A Charakida et al.The British journal of dermatology, 157(3), 569-574 (2007-07-20)
Design and evaluation of a specific, bi-phase extended release system based on differently coated mini-tablets.Aleksandar Aleksovski et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 75, 114-122 (2015-04-08)
Cellulose acetate phthalate microparticles containing Vibrio cholerae: steps toward an oral cholera vaccine.Marta Pastor et al.Journal of drug targeting, 22(6), 478-487 (2014-04-16)
Influence of spray drying manufacturing parameters on quality of losartan potassium microspheres.Andrzej Jankowski et al.Acta poloniae pharmaceutica, 71(5), 833-841 (2014-11-05)
Food Additives, 682-682 (1999)
Formulation and statistical optimization of a novel crosslinked polymeric anti-tuberculosis drug delivery system.Lisa Claire du Toit et al.Journal of pharmaceutical sciences, 97(6), 2176-2207 (2007-09-21)
Evaluating the process parameters of the dry coating process using a 2(5-1) factorial design.Caroline Désirée Kablitz et al.Pharmaceutical development and technology, 18(1), 39-45 (2011-08-20)
Tablets compressed with gastric floating pellets coated with acrylic resin for gastro retention and sustained release of famotidine: in-vitro and in-vivo study.Xiaole Qi et al.The Journal of pharmacy and pharmacology, 67(4), 493-500 (2014-12-17)
Novel in situ gelling ocular films for the opioid growth factor-receptor antagonist-naltrexone hydrochloride: fabrication, mechanical properties, mucoadhesion, tolerability and stability studies.Hamdy Abdelkader et al.International journal of pharmaceutics, 477(1-2), 631-642 (2014-12-03)
Locomotor-reducing effects and structural characteristics of inhaled zerumbone and tetrahydrozerumbone derivatives.Kakuyou Ogawa et al.Biological & pharmaceutical bulletin, 37(9), 1559-1563 (2014-09-02)
Design of a combined process for the inactivation of Salmonella Enteritidis in liquid whole egg at 55°C.S Monfort et al.International journal of food microbiology, 145(2-3), 476-482 (2011-02-19)
Investigation of the drug release and surface morphological properties of film-coated pellets, and physical, thermal and mechanical properties of free films as a function of various curing conditions.Sushmita Bhattacharjya et al.AAPS PharmSciTech, 9(2), 449-457 (2008-04-24)
Eudragit(®) RS PO/RL PO as rate-controlling matrix-formers via roller compaction: Influence of formulation and process variables on functional attributes of granules and tablets.Vivek S Dave et al.Drug development and industrial pharmacy, 38(10), 1240-1253 (2012-01-20)
Dictionary of Food ingredients, 144-144 (2013)
Osmotic pellet system comprising osmotic core and in-process amorphized drug in polymer-surfactant layer for controlled delivery of poorly water-soluble drug.Nilesh Saindane et al.Journal of pharmaceutical sciences, 101(9), 3169-3179 (2012-03-16)
A flexible-dose dispenser for immediate and extended release 3D printed tablets.Katarzyna Pietrzak et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 96, 380-387 (2015-08-19)
The manufacture and characterisation of hot-melt extruded enteric tablets.Gavin P Andrews et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 69(1), 264-273 (2008-01-01)
Formulation considerations in the design of topical, polymeric film-forming systems for sustained drug delivery to the skin.Kit Frederiksen et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 91, 9-15 (2015-01-18)
Preparation of amorphous solid dispersions by rotary evaporation and KinetiSol Dispersing: approaches to enhance solubility of a poorly water-soluble gum extract.Ryan C Bennett et al.Drug development and industrial pharmacy, 41(3), 382-397 (2013-12-18)
Moisture plasticization for enteric Eudragit® L30D-55-coated pellets prior to compression into tablets.Soravoot Rujivipat et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 81(1), 223-229 (2012-01-25)
A novel approach to a fine particle coating using porous spherical silica as core particles.Makoto Ishida et al.Drug development and industrial pharmacy, 40(8), 1054-1064 (2013-06-21)
Design and study of ibuprofen disintegrating sustained-release tablets comprising coated pellets.M R Abbaspour et al.European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 68(3), 747-759 (2007-11-06)
Characterizations of plasticized polymeric film coatings for preparing multiple-unit floating drug delivery systems (muFDDSs) with controlled-release characteristics.Sheng-Feng Hung et al.PloS one, 9(6), e100321-e100321 (2014-06-27)
Formulation and characterization of ORMOSIL particles loaded with budesonide for local colonic delivery.Vesna Petrovska-Jovanovska et al.International journal of pharmaceutics, 484(1-2), 75-84 (2015-02-25)
Accelerating the dissolution of enteric coatings in the upper small intestine: evolution of a novel pH 5.6 bicarbonate buffer system to assess drug release.Felipe J O Varum et al.International journal of pharmaceutics, 468(1-2), 172-177 (2014-04-15)
Development of taste masked caffeine citrate formulations utilizing hot melt extrusion technology and in vitro-in vivo evaluations.Manjeet B Pimparade et al.International journal of pharmaceutics, 487(1-2), 167-176 (2015-04-19)
Influence of additives on melt viscosity, surface tension, and film formation of dry powder coatings.Dorothea Sauer et al.Drug development and industrial pharmacy, 35(6), 646-654 (2009-04-02)
A study on the applicability of in-line measurements in the monitoring of the pellet coating process.Grega Hudovornik et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 75, 160-168 (2015-05-03)
Enteric protection of naproxen in a fixed-dose combination product produced by hot-melt co-extrusion.A-K Vynckier et al.International journal of pharmaceutics, 491(1-2), 243-249 (2015-06-13)
[Effects of wheat leaf surface waxes on the feeding of two wheat aphid species].Yong Liu et al.Ying yong sheng tai xue bao = The journal of applied ecology, 18(8), 1785-1788 (2007-11-03)
Preparation and evaluation of diltiazem hydrochloride diffusion-controlled transdermal delivery system.Ekapol Limpongsa et al.AAPS PharmSciTech, 9(2), 464-470 (2008-04-24)
Preparation and evaluation of sustained-release matrix tablets based on metoprolol and an acrylic carrier using injection moulding.T Quinten et al.AAPS PharmSciTech, 13(4), 1197-1211 (2012-09-12)
Controlled release of acidic drugs in compendial and physiological hydrogen carbonate buffer from polymer blend-coated oral solid dosage forms.R Wulff et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 77, 246-253 (2015-06-21)
备注
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).
| 名称 | CAS号 |
|---|

相关产品
相关资讯
问
热门标签
询 单
请 填 写 相 关 信 息
- 请填写产品名称!
- CAS号
- 请正确填写公司名称!
- 请正确填写联系人!
- 请正确填写联系电话!
- 请正确填写联系邮箱!
- 请描述您的问题!