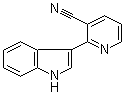
2-Indol-3-ylnicotinonitrile | CAS:3191-30-8
2-Indol-3-ylnicotinonitrile
- 名称:2-吲哚-3-基烟腈 | 2-Indol-3-ylnicotinonitrile
- CAS号:3191-30-8
- 别名:
- 分子式:C14H9N3
- 分子量:219.24
- EINESC号:
产品描述
物理化学性质
| 密度 | 1.31 |
|---|
安全数据
| 没有数据 | 没有数据 |
|---|
SDS
| 来源 | SDS样本 |
|---|---|
| 没有数据 | 没有数据 |
产品合成路线
| 名称 | CAS号 |
|---|
更多
文章
同行评议文献
Involvement of stearoyl-CoA desaturase in the reduction of amidoxime prodrugs.R Reh et al.Xenobiotica; the fate of foreign compounds in biological systems, 38(9), 1177-1190 (2008-07-09)
Genotoxic activities of benzamidine and its N-hydroxylated metabolite benzamidoxime in Salmonella typhimurium and mammalian cells.B Clement et al.Journal of cancer research and clinical oncology, 114(4), 363-368 (1988-01-01)
[Biotransformation of benzamidine and benzamidoxime by microsomal enzymes of the rabbit].B Clement et al.Archiv der Pharmazie, 322(7), 431-435 (1989-07-01)
The fourth molybdenum containing enzyme mARC: cloning and involvement in the activation of N-hydroxylated prodrugs.Sanja Gruenewald et al.Journal of medicinal chemistry, 51(24), 8173-8177 (2008-12-05)
Parallel synthesis of 1,2,4-oxadiazoles using CDI activation.T L Deegan et al.Bioorganic & medicinal chemistry letters, 9(2), 209-212 (1999-02-18)
Microsomal cytochrome P450 dependent oxidation of N-hydroxyguanidines, amidoximes, and ketoximes: mechanism of the oxidative cleavage of their C=N(OH) bond with formation of nitrogen oxides.A Jousserandot et al.Biochemistry, 37(49), 17179-17191 (1998-12-23)
Metabolism of benzamidoxime (N-hydroxyamidine) in human hepatocytes and role of UDP-glucuronosyltransferases.A K Fröhlich et al.Xenobiotica; the fate of foreign compounds in biological systems, 35(1), 17-25 (2005-03-25)
Insight into technetium amidoxime complex: oxo technetium(V) complex of N-substituted benzamidoxime as new basic structure for molecular imaging.Khajadpai Thipyapong et al.Inorganic chemistry, 50(3), 992-998 (2011-01-14)
Isolation and characterization of the protein components of the liver microsomal O2-insensitive NADH-benzamidoxime reductase.B Clement et al.The Journal of biological chemistry, 272(31), 19615-19620 (1997-08-01)
Hepatic, extrahepatic, microsomal, and mitochondrial activation of the N-hydroxylated prodrugs benzamidoxime, guanoxabenz, and Ro 48-3656 ([[1-[(2s)-2-[[4-[(hydroxyamino)iminomethyl]benzoyl]amino]-1-oxopropyl]-4-piperidinyl]oxy]-acetic acid).Bernd Clement et al.Drug metabolism and disposition: the biological fate of chemicals, 33(11), 1740-1747 (2005-08-25)
Characterization and partial purification of the rat and human enzyme systems active in the reduction of N-hydroxymelagatran and benzamidoxime.Susanne Andersson et al.Drug metabolism and disposition: the biological fate of chemicals, 33(4), 570-578 (2005-01-11)
Biotransformation of benzamidine and benzamidoxime in vivo.B Clement et al.Archiv der Pharmazie, 326(10), 807-812 (1993-10-01)
N-hydroxylation of benzamidine to benzamidoxime by a reconstituted cytochrome P-450 oxidase system from rabbit liver: involvement of cytochrome P-450 IIC3.B Clement et al.Molecular pharmacology, 43(3), 335-342 (1993-03-01)
Identification of the missing component in the mitochondrial benzamidoxime prodrug-converting system as a novel molybdenum enzyme.Antje Havemeyer et al.The Journal of biological chemistry, 281(46), 34796-34802 (2006-09-16)
Characteristics of the microsomal N-hydroxylation of benzamidine to benzamidoxime.B Clement et al.Xenobiotica; the fate of foreign compounds in biological systems, 17(6), 659-667 (1987-06-01)
Metabolic N-hydroxylation of pentamidine in vitro.B J Berger et al.Antimicrobial agents and chemotherapy, 34(9), 1678-1684 (1990-09-01)
Reduction of N(ω)-hydroxy-L-arginine by the mitochondrial amidoxime reducing component (mARC).Jürke Kotthaus et al.The Biochemical journal, 433(2), 383-391 (2010-10-30)
Phase 2 metabolites of N-hydroxylated amidines (amidoximes): synthesis, in vitro formation by pig hepatocytes, and mutagenicity testing.B Clement et al.Chemical research in toxicology, 14(3), 319-326 (2001-03-22)
A novel and specific fluorescence reaction for uracil.Takayuki Shibata et al.Analytica chimica acta, 674(2), 234-238 (2010-08-04)
Sensitive and Selective Determination of Orotic Acid in Biological Specimens Using a Novel Fluorogenic Reaction.Sheng Yin et al.Journal of fluorescence, 25(4), 1005-1011 (2015-06-01)
Reduction of amphetamine hydroxylamine and other aliphatic hydroxylamines by benzamidoxime reductase and human liver microsomes.B Clement et al.Chemical research in toxicology, 13(10), 1037-1045 (2000-11-18)
备注
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).
| 名称 | CAS号 |
|---|

相关产品
相关资讯
问
热门标签
询 单
请 填 写 相 关 信 息
- 请填写产品名称!
- CAS号
- 请正确填写公司名称!
- 请正确填写联系人!
- 请正确填写联系电话!
- 请正确填写联系邮箱!
- 请描述您的问题!