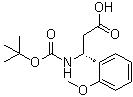
(betaR)-beta-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-methoxybenzenepropanoic acid | CAS:500788-85-2
(betaR)-beta-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-methoxybenzenepropanoic acid
- 名称:(betaR)-beta-[[叔丁氧羰基]氨基]-2-甲氧基苯丙酸 | (betaR)-beta-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-methoxybenzenepropanoic acid
- CAS号:500788-85-2
- 别名:
- 分子式:C15H21NO5
- 分子量:295.33
- EINESC号:
产品描述
物理化学性质
| 密度 | 1.167±0.06 g/cm3 (20 º |
|---|
安全数据
| 没有数据 | 没有数据 |
|---|
SDS
| 来源 | SDS样本 |
|---|---|
| 没有数据 | 没有数据 |
产品合成路线
| 名称 | CAS号 |
|---|
更多
文章
同行评议文献
Solventless visible light-curable coating: II. Drug release, mechanical strength and photostability.Sagarika Bose et al.International journal of pharmaceutics, 393(1-2), 41-47 (2010-04-08)
Formulation development and in vitro evaluation of solidified self-microemulsion in the form of tablet containing atorvastatin calcium.Kazi Asraf Ali et al.Drug development and industrial pharmacy, 39(11), 1742-1749 (2012-12-12)
Development and optimization of dextromethorphan hydrobromide oral disintegrating tablets: effect of formulation and process variables.Haitham Fady Mostafa et al.Pharmaceutical development and technology, 18(2), 454-463 (2012-08-14)
Physicochemical interactions between drugs and superdisintegrants.Nelly Fransén et al.The Journal of pharmacy and pharmacology, 60(12), 1583-1589 (2008-11-13)
[Comparative evaluation of the anionic superdisintegrant incorporation mode on the quality of ranitidine tablets].Liliana Postolache et al.Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi, 116(1), 336-340 (2012-10-20)
Effect of starch 1500 as a binder and disintegrant in lamivudine tablets prepared by high shear wet granulation.Bytul M Rahman et al.Pakistan journal of pharmaceutical sciences, 21(4), 455-459 (2008-10-22)
Design, development and optimization of a novel time and pH-dependent colon targeted drug delivery system.Mayur M Patel et al.Pharmaceutical development and technology, 14(1), 62-69 (2008-09-20)
Effect of disintegrants on the properties of multiparticulate tablets comprising starch pellets and excipient granules.Samata Mehta et al.International journal of pharmaceutics, 422(1-2), 310-317 (2011-11-22)
An easy-to-use approach for determining the disintegration ability of disintegrants by analysis of available surface area.Yasunori Iwao et al.International journal of pharmaceutics, 448(1), 1-8 (2013-03-23)
Ultrasonic approach for viscoelastic and microstructure characterization of granular pharmaceutical tablets.Armin Saeedi Vahdat et al.International journal of pharmaceutics, 454(1), 333-343 (2013-07-04)
Pulsatile drug delivery to ileo-colonic segments by structured incorporation of disintegrants in pH-responsive polymer coatings.R C A Schellekens et al.Journal of controlled release : official journal of the Controlled Release Society, 132(2), 91-98 (2008-09-09)
Water content determination of superdisintegrants by means of ATR-FTIR spectroscopy.G Szakonyi et al.Journal of pharmaceutical and biomedical analysis, 63, 106-111 (2012-03-01)
II. Technological approaches to improve the dissolution behavior of nateglinide, a lipophilic insoluble drug: co-milling.Lauretta Maggi et al.International journal of pharmaceutics, 454(1), 568-572 (2013-07-23)
Functional assessment of four types of disintegrants and their effect on the spironolactone release properties.John Rojas et al.AAPS PharmSciTech, 13(4), 1054-1062 (2012-08-18)
Preparation of cross-linked carboxymethyl jackfruit starch and evaluation as a tablet disintegrant.Nisit Kittipongpatana et al.Pakistan journal of pharmaceutical sciences, 24(4), 415-420 (2011-10-01)
Zero-order release profile of metoclopramide hydrochloride sublingual tablet formulation.Randa Latif Pharmaceutical development and technology, 18(6), 1372-1378 (2012-09-12)
In vitro determination of aceclofenac Mouth Dissolving Tablets.Shobhit Shobhit et al.Polimery w medycynie, 43(4), 227-229 (2014-03-07)
Enhanced dissolution and bioavailability of raloxifene hydrochloride by co-grinding with different superdisintegrants.Balasubramaniam Jagadish et al.Chemical & pharmaceutical bulletin, 58(3), 293-300 (2010-03-02)
Solventless visible light-curable coating: I. Critical formulation and processing parameters.Sagarika Bose et al.International journal of pharmaceutics, 393(1-2), 32-40 (2010-02-09)
Development and evaluation of fast-dissolving tablets of meloxicam-β-cyclodextrin complex prepared by direct compression.Aiman A Obaidat et al.Acta pharmaceutica (Zagreb, Croatia), 61(1), 83-91 (2011-03-17)
Formulation and evaluation of swellable and floating gastroretentive ciprofloxacin hydrochloride tablets.Ramji Anil Kumar Arza et al.AAPS PharmSciTech, 10(1), 220-226 (2009-03-12)
Dissolution rate enhancement of gliclazide by ordered mixing.Vikas A Saharan et al.Acta pharmaceutica (Zagreb, Croatia), 61(3), 323-334 (2011-09-29)
A comparison of chitosan-silica and sodium starch glycolate as disintegrants for spheronized extruded microcrystalline cellulose pellets.Alvaro Goyanes et al.Drug development and industrial pharmacy, 37(7), 825-831 (2011-03-17)
Evaluation of physico-mechanical properties of drug-excipients agglomerates obtained by crystallization.M Maghsoodi et al.Pharmaceutical development and technology, 16(3), 243-249 (2010-02-24)
Application of face centred central composite design to optimise compression force and tablet diameter for the formulation of mechanically strong and fast disintegrating orodispersible tablets.Ritesh M Pabari et al.International journal of pharmaceutics, 430(1-2), 18-25 (2012-04-03)
备注
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).
| 名称 | CAS号 |
|---|

相关产品
相关资讯
问
热门标签
询 单
请 填 写 相 关 信 息
- 请填写产品名称!
- CAS号
- 请正确填写公司名称!
- 请正确填写联系人!
- 请正确填写联系电话!
- 请正确填写联系邮箱!
- 请描述您的问题!