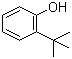
2-tert-Butylphenol | CAS:88-18-6
2-tert-Butylphenol
- 名称:2-叔丁基苯酚; 邻叔丁基苯酚 | 2-tert-Butylphenol
- CAS号:88-18-6
- 别名:
- 分子式:C10H14O
- 分子量:150.22
- EINESC号:201-807-2
产品描述
物理化学性质
| 熔点 | -7 ºC |
|---|---|
| 水溶性 | 0.23 g/100 mL (20 ºC) |
| 沸点 | 224 ºC |
| 闪点 | 110 ºC |
| 密度 | 0.978 |
| 折射率 | 1.522-1.524 |
安全数据
| 危险品标志 | T;N |
|---|---|
| 危险类别码 | R22;R23;R34;R51/53 |
| 安全说明书 | S26;S36/37/39;S45;S61 |
SDS
| 来源 | SDS样本 |
|---|---|
| 没有数据 | 没有数据 |
产品合成路线
| 名称 | CAS号 |
|---|
更多
文章
同行评议文献
Physico-chemical comparison of betulinic acid, betulin and birch bark extract and in vitro investigation of their cytotoxic effects towards skin epidermoid carcinoma (A431), breast carcinoma (MCF7) and cervix adenocarcinoma (HeLa) cell lines.Codruta M Soica et al.Natural product research, 26(10), 968-974 (2011-05-21)
Extraction of betulin, trimyristin, eugenol and carnosic acid using water-organic solvent mixtures.Fulgentius N Lugemwa Molecules (Basel, Switzerland), 17(8), 9274-9282 (2012-08-07)
A key regulator of cholesterol homoeostasis, SREBP-2, can be targeted in prostate cancer cells with natural products.James R Krycer et al.The Biochemical journal, 446(2), 191-201 (2012-06-05)
alpha-Glucosidase inhibitory activity of triterpenoids from Cichorium intybus.Atta-ur-Rahman et al.Journal of natural products, 71(5), 910-913 (2008-03-18)
[Chemical constituents of Dolomiaea souliei].Hu Wei et al.Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 37(9), 1249-1253 (2012-07-19)
Betulinol and wood sterols in sediments contaminated by pulp and paper mill effluents: dissolution and spatial distribution.H Ratia et al.Environmental science and pollution research international, 20(7), 4562-4573 (2012-12-25)
Cytotoxic betulin-derived hydroxypropargylamines trigger apoptosis.René Csuk et al.Bioorganic & medicinal chemistry, 21(2), 425-435 (2012-12-19)
alpha-Glucosidase inhibitory activity of triterpenoids from Cichorium intybus.Atta-ur-Rahman et al.Journal of natural products, 71(5), 910-913 (2008-03-18)
A new cycloartane nortriterpenoid from stem and leaf of Quercus variabilis.Ling-Yun Jia et al.Journal of Asian natural products research, 15(9), 1050-1054 (2013-07-23)
Extraction of betulin, trimyristin, eugenol and carnosic acid using water-organic solvent mixtures.Fulgentius N Lugemwa Molecules (Basel, Switzerland), 17(8), 9274-9282 (2012-08-07)
A key regulator of cholesterol homoeostasis, SREBP-2, can be targeted in prostate cancer cells with natural products.James R Krycer et al.The Biochemical journal, 446(2), 191-201 (2012-06-05)
Simultaneous quantification of five marker compounds of Betula utilis stem bark using a validated high-performance thin-layer chromatography method.Imran Khan et al.Journal of separation science, 35(3), 392-399 (2012-01-04)
Antidiabetic property of Aerva lanata (L.) Juss. ex Schult. is mediated by inhibition of alpha glucosidase, protein glycation and stimulation of adipogenesis.Mariam Philip Riya et al.Journal of diabetes, 7(4), 548-561 (2014-09-17)
Simultaneous quantification of five marker compounds of Betula utilis stem bark using a validated high-performance thin-layer chromatography method.Imran Khan et al.Journal of separation science, 35(3), 392-399 (2012-01-04)
Betulin-derived compounds as inhibitors of alphavirus replication.Leena Pohjala et al.Journal of natural products, 72(11), 1917-1926 (2009-10-21)
Srebf1 Controls Midbrain Dopaminergic Neurogenesis.Enrique M Toledo et al.Cell reports, 31(5), 107601-107601 (2020-05-07)
Chemical constituents of Caesalpinia decapetala (Roth) Alston.Xiao-Hua Wei et al.Molecules (Basel, Switzerland), 18(1), 1325-1336 (2013-01-24)
Haemolytic activity, cytotoxicity and membrane cell permeabilization of semi-synthetic and natural lupane- and oleanane-type saponins.Charles Gauthier et al.Bioorganic & medicinal chemistry, 17(5), 2002-2008 (2009-02-10)
A bicentennial of betulin.Hayek EWH, et al. Phytochemistry, 28(9), 2229-2242 (1989)
Efficient synthesis and biological evaluation of epiceanothic acid and related compounds.Pu Zhang et al.Bioorganic & medicinal chemistry letters, 21(1), 338-341 (2010-12-03)
Betulin, betulinic acid and butein are inhibitors of acetaldehyde-induced activation of liver stellate cells.Agnieszka Szuster-Ciesielska et al.Pharmacological reports : PR, 63(5), 1109-1123 (2011-12-20)
A novel one-step microbial transformation of betulin to betulinic acid catalysed by Cunninghamella blakesleeana.Yu Feng et al.Food chemistry, 136(1), 73-79 (2012-09-29)
Triterpenes involved in the anti-inflammatory effect of ethanolic extract of Pterodon emarginatus Vogel stem bark.Weuller F de Moraes et al.Journal of natural medicines, 66(1), 202-207 (2011-06-07)
Betulin-betulinic acid natural product based analogs as anti-cancer agents.Subash C Jonnalagadda et al.Anti-cancer agents in medicinal chemistry, 13(10), 1477-1499 (2013-07-16)
Synthesis and cytotoxic activity of new betulin and betulinic acid esters with conjugated linoleic acid (CLA).Barbara Tubek et al.Natural product communications, 8(4), 435-438 (2013-06-07)
Antiproliferative triterpenes from Melaleuca ericifolia.Fatma M Abdel Bar et al.Journal of natural products, 71(10), 1787-1790 (2008-10-02)
Intrinsically microporous polyesters from betulin - toward renewable materials for gas separation made from birch bark.Jekaterina Jeromenok et al.Macromolecular rapid communications, 32(22), 1846-1851 (2011-09-20)
Cytotoxic heterocyclic triterpenoids derived from betulin and betulinic acid.Milan Urban et al.Bioorganic & medicinal chemistry, 20(11), 3666-3674 (2012-05-04)
Betulin and its derivatives. Chemistry and biological activity.Tolstikov GA, et al. Chemistry for Sustainable Development, 13, 1-29 (2005)
Novel betulin derivatives as antileishmanial agents with mode of action targeting type IB DNA topoisomerase.Sayan Chowdhury et al.Molecular pharmacology, 80(4), 694-703 (2011-07-14)
A concise semi-synthetic approach to betulinic acid from betulin.Kim DSHL, et al. Synthetic Communications, 27(9), 1607-1612 (1997)
Correlation of cytotoxic activity of betulinines and their hydroxy analogues.Jan Sarek et al.Bioorganic & medicinal chemistry letters, 15(19), 4196-4200 (2005-07-30)
Pharmacological properties of the ubiquitous natural product betulin.Sami Alakurtti et al.European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 29(1), 1-13 (2006-05-24)
Chemical Constituents of the Roots of Euphorbia micractina.Wendong Xu et al.Journal of natural products, 72(9), 1620-1626 (2009-08-26)
Alkylidene branched lupane derivatives: synthesis and antitumor activity.René Csuk et al.European journal of medicinal chemistry, 53, 337-345 (2012-05-15)
Betulin as an antitumor agent tested in vitro on A431, HeLa and MCF7, and as an angiogenic inhibitor in vivo in the CAM assay.Cristina Adriana Dehelean et al.Natural product communications, 7(8), 981-985 (2012-09-18)
Study of skin anti-ageing and anti-inflammatory effects of dihydroquercetin, natural triterpenoids, and their synthetic derivatives.Chan Woo Lee et al.Bioorganicheskaia khimiia, 38(3), 374-381 (2012-09-25)
Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes.Cédric Genet et al.Journal of medicinal chemistry, 53(1), 178-190 (2009-11-17)
Naturally occurring pentacyclic triterpenes as inhibitors of glycogen phosphorylase: synthesis, structure-activity relationships, and X-ray crystallographic studies.Xiaoan Wen et al.Journal of medicinal chemistry, 51(12), 3540-3554 (2008-06-04)
Cytotoxicity of artesunic acid homo- and heterodimer molecules toward sensitive and multidrug-resistant CCRF-CEM leukemia cells.Cindy Horwedel et al.Journal of medicinal chemistry, 53(13), 4842-4848 (2010-06-10)
Chemical constituents and allelopathic and antioxidant activities of Alchorneopsis floribunda Müll. Arg. (Euphorbiaceae).Elenilze Figueiredo Batista et al.Natural product research, 27(1), 1-8 (2011-12-14)
Antitrypanosomal activity of triterpenoids and sterols from the leaves of Strychnos spinosa and related compounds.Sara Hoet et al.Journal of natural products, 70(8), 1360-1363 (2007-07-20)
Distribution and expression characteristics of triterpenoids and OSC genes in white birch (Betula platyphylla suk.).Jing Yin et al.Molecular biology reports, 39(3), 2321-2328 (2011-06-08)
Lupane triterpenoids from Maytenus species.Marvin J Núñez et al.Journal of natural products, 68(7), 1018-1021 (2005-07-26)
A comparison investigation on the solubilization of betulin and betulinic acid in cyclodextrin derivatives.Hai Ming Wang et al.Natural product communications, 7(3), 289-291 (2012-05-02)
Synthesis, cytotoxicity, and haemolytic activity of chacotrioside lupane-type neosaponins and their germanicane-type rearrangement products.Charles Gauthier et al.Bioorganic & medicinal chemistry letters, 19(8), 2310-2314 (2009-03-17)
A practical synthesis of betulonic acid using selective oxidation of betulin on aluminium solid support.Nina Melnikova et al.Molecules (Basel, Switzerland), 17(10), 11849-11863 (2012-10-23)
Identification of pentacyclic triterpenes derivatives as potent inhibitors against glycogen phosphorylase based on 3D-QSAR studies.Zhongjie Liang et al.European journal of medicinal chemistry, 46(6), 2011-2021 (2011-03-29)
Study of the betulin molecule in a water environment; ab initio and molecular simulation calculations.Miroslav Pospíšil et al.Journal of molecular modeling, 18(1), 367-376 (2011-05-04)
A new cycloartane nortriterpenoid from stem and leaf of Quercus variabilis.Ling-Yun Jia et al.Journal of Asian natural products research, 15(9), 1050-1054 (2013-07-23)
Synthesis and cytotoxicity of bidesmosidic betulin and betulinic acid saponins.Charles Gauthier et al.Journal of natural products, 72(1), 72-81 (2009-01-01)
备注
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).
| 名称 | CAS号 |
|---|

相关产品
相关资讯
问
热门标签
询 单
请 填 写 相 关 信 息
- 请填写产品名称!
- CAS号
- 请正确填写公司名称!
- 请正确填写联系人!
- 请正确填写联系电话!
- 请正确填写联系邮箱!
- 请描述您的问题!